
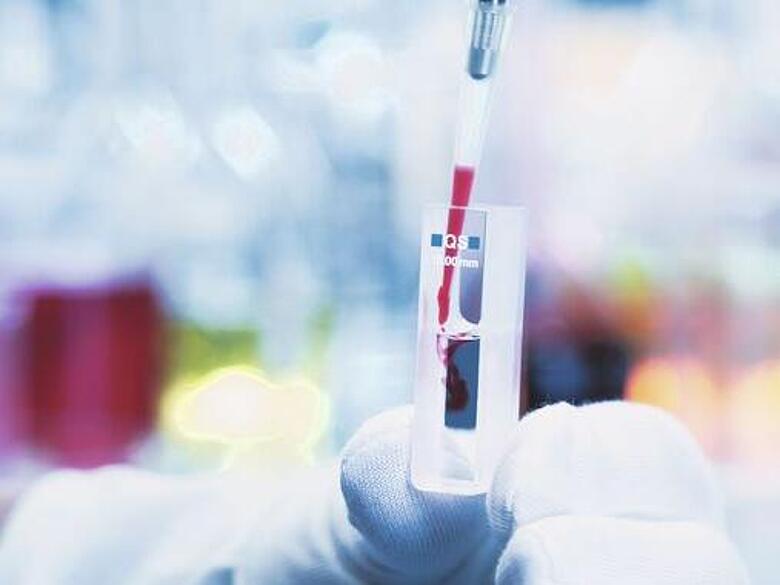
Hellma Analytics produces a wide range of cuvettes for use in spectroscopy and cytometry. Thanks to their stability, maximum precision and reliability when used for absorbance, fluorescence and Raman measurements, Hellma cuvettes, flow-through cuvettes and probes work exceptionally well in a wide range of areas in the lab.
Our practical cuvette finder supports you in finding the right cuvette for your individual application. Indicate the relevant properties and in no time you will find the right product from the world’s largest range of glass and quartz glass cuvettes.
By entering specific operating conditions in our helpful Optical Probes and Flow Cell Configurator, you can find the right laboratory probe or the right laboratory measuring cell for your needs. If you cannot find the right item in the preconfigured product area, you can use our configurator to customize your probe or measuring cell or put them together from scratch.

In our calibration laboratory, which is accredited* by DAkkS according to DIN EN ISO/IEC 17025, we manufacture certified reference materials based on the regulatory issued by NIST (National Institute of Standards and Technology), ASTM (American Society for Testing and Materials) and Pharmacopeias (Ph. Eur., USP). As a result, our certified UV / Vis reference materials meet international standards and create the basis for reliable measurements for our customers.
*) The accreditation is valid only for the scope listed in the annex of the accreditation certificate D-K-18752-01-00

